|
Absolute age dating determines how many years before the present a rock
formed. It gives the age of the rock in years. Geologists use radioactive
elements to do absolute age dating. Radioactive elements are
unstable. Over time, they decay or change into stable elements or will
decay to become either some nearby element in the periodic table or some
isotope of the same element. For example, radioactive potassium-40
decays into calcium-40 (11%) and Argon-40 (89%). We know how long it
takes for such a transformation, by calculating the amount of daughter
material (calcium and argon) that is produced from potassium. The
time it takes is called its ``half_life'' and represents the
average time an atom will survive in its original state.
Radiometric clocks are "set" when each rock is created. So
the moment an igneous rock solidifies from magma or a sedimentary rock
layer is deposited or a rock changed by metamorphism, the clock begins.
It is this process that gives us the ability to date rocks that formed
at different times in earth history.
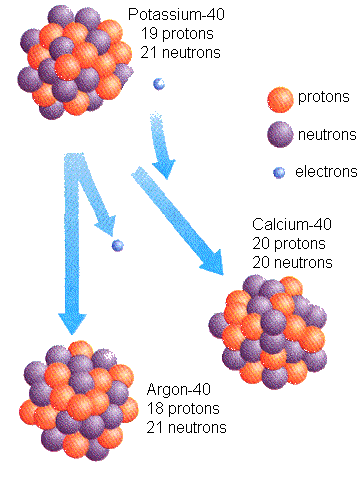
Radioactive decay of Potassium-40
|