Water Cycle, Weather, Climate over Time
LEARNING HOW WATER DISSOLVES SUBSTANCES
|
OBJECTIVES:
VOCABULARY:
·
clean
MATERIALS: BACKGROUND:
Water is a universal solvent which means it can dissolve many other
substances within the molecular structure of water.
Water becomes salty because many different components that erode
from the land will dissolve and become part of the water.
Over eons of time the water cycle evaporates only fresh water,
leaving the “salts” behind.
The term solution means a system in which one or more substances are
uniformly mixed or dissolved in another substance.
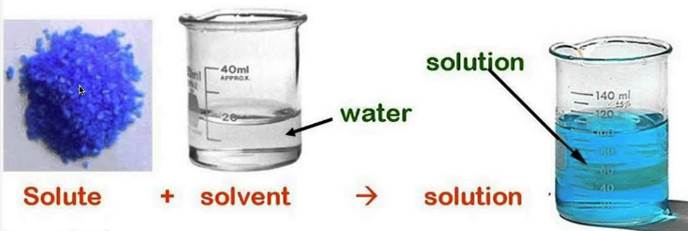
A solution has two components, a solute and a solvent.
The solute is the substance that is dissolved.
The solvent is the substance doing the dissolving.
A solute plus a solvent equals a solution.
Water is considered a universal solvent, in other words, many other
substances can be dissolved into water.
Seawater is an example, it contains many ions of dissolved elements
like sodium, chlorine, bromine, calcium, carbon, and many more.
Seawater starts as fresh water but as water falls on the land
causing the erosion of rocks, minerals become a part of the water, and
then become part of seawater.
Salt water is neither dirty nor polluted, it is a solution that is
clean, unless polluted by humans or nature.
The students should remember that the hydrogen and oxygen "bond" together,
or "hold hands." The bond is
very strong and is called a covalent bond. Because the bond is so strong,
water is considered a universal solvent, since many things dissolve in it.
Water is a special type of covalent bond called a hydrogen bond.
Salts on the other hand hold hands very weakly and break up very
easily in water. This is
called an ionic bond.
The break up of salts in water causes the water to have the ions of that
salt. For instance,
table salt is sodium chloride (NaCl).
When it is dissolved in water it turns into a positive ion of
sodium (Na+) and a negative ion of chlorine (Cl-).
Dissolving does not mean that the compound breaks into its
elements. If that was the
case, sodium, the element is reactive with water and chlorine is a deadly
gas. It is important to use
the correct terms early in a student’s education, so they don't get
confused later on.
The term mixture can also be confusing.
There are two types homogeneous and heterogeneous.
Homogeneous mixtures appear
uniform to the eye. The chemical composition is the same for any sample of
the mixture. When materials
dissolve they produce a homogeneous mixture.
The solute breaks down and is spread throughout the solvent at a
molecular level (which is why the solute is no longer visible).
Heterogeneous mixtures are
not uniform. If you take two samples from different parts of the mixture,
they will not have an identical composition.
When things do not dissolve, the mixture is not uniform.
Mixtures (whether homogenous or heterogeneous) do
not produce a chemical change—that is, if you remove the solvent (in this
case water), from a mixture, the resulting material will be the same as
the solute (for example, if you remove water from salt water, you will get
salt again).
PROCEDURE:
1. Ask students if a body of salt water were to
evaporate, would anything be left. If the students debate over this
you might want to set out a dish of salt water and a dish of fresh water
and have them observe what happens when the water evaporates. You
can prepare this ahead of time to show them salt residue in a dish after
evaporation of salt water.
Use the powerpoint
presentation and go over Solute, Solution, Solvent, and Mixture.
Dissolve---
Solids, liquids, and gases can all dissolve. Dissolving
depends on the molecules of the substance doing the dissolving,called
the solvent, and the molecules of the substance being dissolved, called
the solute. Dissolving is the process in which these molecules
interact and attract each other to form a solution.
When you introduce the
materials make sure you go over each one. For example: water
is the solvent + salt is the solute = results in a solution that is
salty.
In all cases, students will
be making mixture. In some
cases the material will dissolve making a homogeneous mixture, whereas in
others the solute will not dissolve producing a heterogeneous mixture.
2. In this lab, the students will measure all the
correct amounts and follow the lab sheet. Have them describe what
happens when they mix the materials. Once the students record their
findings on one item, have them clean out the dish and proceed to the next
item.
If the material dissolves,
it will spread throughout the water and the solid material will disappear.
Students should record whether the materials dissolve or not (sand
and mud do not).
Students should time how
long the material takes to dissolve so they can see which material
dissolves the fastest and whether materials dissolve faster in warm or
cool water. Students can watch
the class clock or just count seconds and write down the time for each.
(When the solid crystals are no
longer visible, they should stop counting).
Overall, all the solutes
dissolve faster in the warm water than cool but there is variability in
the rate between the solutes that dissolve.
At the end do NOT
dispose of the sand and mud in the sink. You may want to save the
sand and dry it out, to be used again. 3. Students may ask you why the oceans aren't sweet. The oceans are not sweet because sugar does not dissolve as fast as salt and does not stay dissolved. Sugar has strong bonds (covalent) whereby salts have weak bonds (ionic). Also, salts are much more abundant in the rocks. Have you ever tasted a sweet rock?
|